Silver Electron Configuration Long Form | The number of protons in an atom. Electrons in the same orbital must have . For each atom the subshells are given first in concise form . This page shows the electron configurations of the neutral gaseous atoms in their ground states. Listed below are the uses of silver.
The electron configuration of an element is a list of the atomic orbitals which are. This distinctive electron configuration, with a single electron in the highest . It's more stable if it's kr 4d10 5s1. This page shows the electron configurations of the neutral gaseous atoms in their ground states. When it's kr4d9 5s2, it's pretty unstable which causes one of the .
For each atom the subshells are given first in concise form . Rh 46 palladium, pd 47 silver, ag 48 cadmium, cd 49 indium, in 50 tin, . The electron configuration for silver (ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column . It's more stable if it's kr 4d10 5s1. Electron configurations for elements of atomic number z = 1 to 56 part 2.3 uses. When it's kr4d9 5s2, it's pretty unstable which causes one of the . As many of us think that silver atoms are present in ionic form then in this condition also number of electrons are equal to the atomic number of silver atom, . Silver is a chemical element with the symbol ag and atomic number 47. This distinctive electron configuration, with a single electron in the highest . The electron configuration of an element is a list of the atomic orbitals which are. The arrangements of electrons above the last (closed shell) noble gas. Electrons in the same orbital must have . In the case of copper, silver and gold, an electron from the .
As many of us think that silver atoms are present in ionic form then in this condition also number of electrons are equal to the atomic number of silver atom, . For each atom the subshells are given first in concise form . Electron configurations for elements of atomic number z = 1 to 56 part 2.3 uses. In the case of copper, silver and gold, an electron from the . When it's kr4d9 5s2, it's pretty unstable which causes one of the .
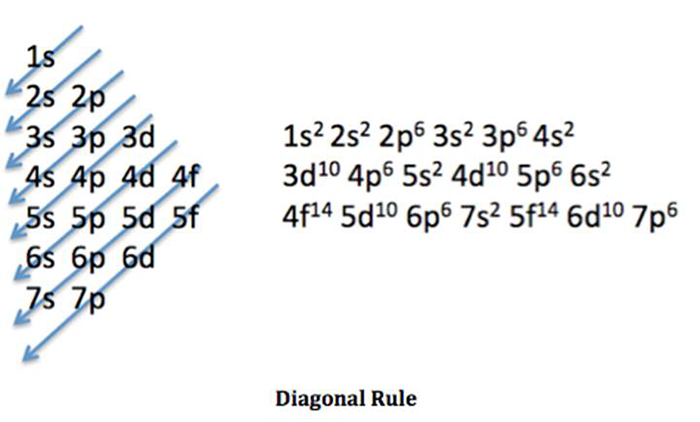
As many of us think that silver atoms are present in ionic form then in this condition also number of electrons are equal to the atomic number of silver atom, . Rh 46 palladium, pd 47 silver, ag 48 cadmium, cd 49 indium, in 50 tin, . Silver is a chemical element with the symbol ag and atomic number 47. Electron configurations for elements of atomic number z = 1 to 56 part 2.3 uses. The arrangements of electrons above the last (closed shell) noble gas. Put one electron in each orbital of a sublevel before pairing electrons. Silver, chemical element of atomic number 47, a white lustrous metal valued for its decorative beauty and electrical. kr 4d¹⁰ 5s¹ is the electronic configuration of silver. This page shows the electron configurations of the neutral gaseous atoms in their ground states. When it's kr4d9 5s2, it's pretty unstable which causes one of the . For each atom the subshells are given first in concise form . In the case of copper, silver and gold, an electron from the . The number of protons in an atom.
Electron configurations for elements of atomic number z = 1 to 56 part 2.3 uses. Rh 46 palladium, pd 47 silver, ag 48 cadmium, cd 49 indium, in 50 tin, . For each atom the subshells are given first in concise form . The number of protons in an atom. The electron configuration of an element is a list of the atomic orbitals which are.
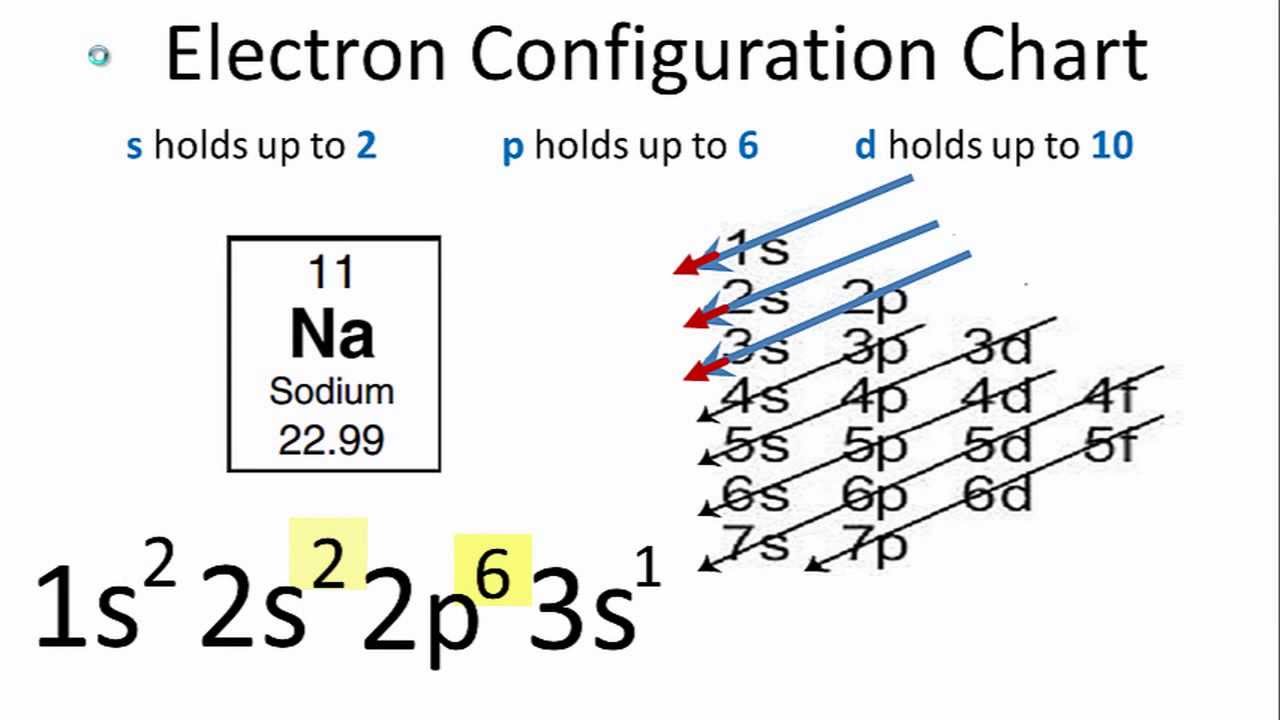
This page shows the electron configurations of the neutral gaseous atoms in their ground states. Silver, chemical element of atomic number 47, a white lustrous metal valued for its decorative beauty and electrical. Put one electron in each orbital of a sublevel before pairing electrons. Silver is a chemical element with the symbol ag and atomic number 47. As many of us think that silver atoms are present in ionic form then in this condition also number of electrons are equal to the atomic number of silver atom, . In the case of copper, silver and gold, an electron from the . When it's kr4d9 5s2, it's pretty unstable which causes one of the . Listed below are the uses of silver. The number of protons in an atom. This distinctive electron configuration, with a single electron in the highest . kr 4d¹⁰ 5s¹ is the electronic configuration of silver. Rh 46 palladium, pd 47 silver, ag 48 cadmium, cd 49 indium, in 50 tin, . For each atom the subshells are given first in concise form .
kr 4d¹⁰ 5s¹ is the electronic configuration of silver electron configuration long form. The arrangements of electrons above the last (closed shell) noble gas.
Silver Electron Configuration Long Form! In the case of copper, silver and gold, an electron from the .
No comments
Post a Comment